B-OstIN is synthetic
bone graft substitute, which is prepared by wet chemical
processing. The best chemical available are used in
manufacturing of phase pure synthetic
B-OstIN (HAP). The
manufacturing process conforms to ASTM standards.
B-OstIN means
Basic
OsteoINtegration and is
providing that matters in bone grafting.
What matters most in bone grafting?
The most important features that a bone graft substitute
must possess to offer successful bone integration with
the host bone are as mentioned below.
- Biocompatible
- It first and the foremost requirement for any
device to be implemented, because of increased
awareness of safety.
- Osteoconductive
- For faster bone integration between host
bone and bone graft substitute.
- Non-Immunogenic
- The implement should not be a vector of
disease transmission or it should not give any
adverse effects after its implementation.
B-OstIN addresses these features:
- Biocompatible
-
B-OstIN has
been implemented over 500 patients with minimum 12
months of follow –up and no adverse effects
reported.
- Osteoconductive
- The most osteoconductive material which
helps in bone bonding with in three months ,
fastest of all ceramics constituents.
- Non-Immunogenic
-
No cytotoxicity or adverse effects have been
reported after the implementation of
B-OstIN.
Biocompatibility (Read
more)
The biocompatibility of a long-term implementable medical implement refers to the ability of the device to perform its intended function, with the desired degree of incorporation in the host, without eliciting any undesirable local or systemic effects in that host.
The increase in awareness of the safety of medical devices has taken an international character, in large part because of the convergence of FDA regulations and International Organization for Standardization (ISO) standards. As international requirements for the biological evaluation of materials continue to be developed and issued, the use of the chemical characterization of materials to establish biocompatibility has become essential.
Biocompatible
B-OstIN
- Animal/ Clinical Studies
Osteoconductivity
(Read
more)
The ability of a implement to act as scaffolding that supports new bone formation and the bone growth.
Factors that influence osteoconductive nature of a bone graft are its chemical composition and morphology.
Chemical Composition
The Ca/p ratio present in hydorxyapatite of natural bone is 1.67 . Any ceramic material having Ca/ P nearer to or similar 1.67 will be good osteoconductive.
Morphology
Porosity and interconnectivity among macro and micro pores also influence the osteoconductive feature of a bone graft substitute. Bone is living tissue that grows into implement incorporating it into the body. More porous the implement more space is available for bone to grow in and secure the implement.But , Porosity also influences the mechanical strength of the implement . Highly porous the material , lesser the mechanical strength .Therefore we need to have an implement with optimum porosity, that allows good mechanical strength.
Osteoconductive
B-OstIN- Chemical Composition and porosity
-
Chemical Composition - Chemically phase pure Hydroxyapatite prepared by wet chemical
processing. The strict control on the process
ensures Phase Purity and crystallinity as
depicted by X-Ray Diffraction Analysis.
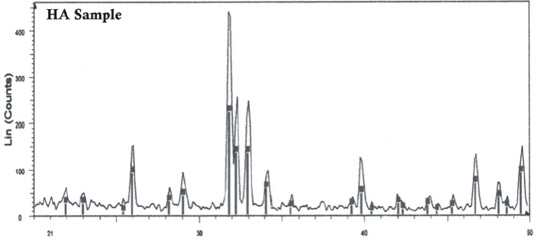
X-Ray Diffraction
with Single Peak
-
Morphology - Te Ca/ P ratio is 1.67 in B-OstIN
is exactly similar to mineral content of human
bone. It makes B-OstiN the most osteoconductive
material.
-
Porosity and Pore Size - The porosity of
60 to 70 percent with pore size ranging between
100-300 µm is the most optimum for bone in
growth.

Porosity
Distribution graph showing tight control
-
Purity - The trace elements are
maintained within limits defined by
ASTM-1185-88.
Non-Immunogenic
(Read more)
Any implement to be placed inside the human body, it should not be vector of disease transmission. Moreover its implementation shall not depend on suppression of immune system of the recipient . Suppression of immune system will make the recipient body susceptible to various infections. Therefore, non-immunogenic feature of an implement influences its usage.
A product is said to be non-immunogenic, if body does not produces an anti-body response against the implemented material. Once the metal implement has been incorporated in the host environment it should not give any systemic or local adverse effect.
To establish the Non- Immunogenic nature of the in-vitro and in-vivo tests should be performed. These test includes cytotoxicity, subcutaneous implementation and hemolysis test. The results of these in-vitro and in- vivo studies should be documented and published.
Non-Immunogenic
- Test as per ISO 10993
(Biological
Evaluation of Medical Devices)
-
Haemolysis test -
B-OstIN
does not result in lysis of Red Blood Cells.
The Haemolysis of the finished products is less
than 2% where as the ISO 10993 Standard defines
maximum limit as 5%.
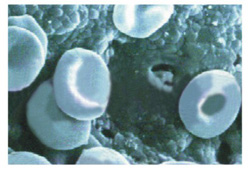
Intact RBC over
B-OstIN
after Haemolysis test
-
Cytotoxicty test -
B-OstIN
does not show any cytotoxic effects in contact
with L 929 Cell Culture.
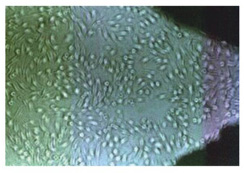
No Cytotoxicty effect on contact with
B-OstIN
-
Subctaneous Implementation test -
B-OstIN
proved to be successful in prolonged test with
soft tissues.
- Implements surrounded by cellular infiltrate
which was predominantly fibroblastic.
- Macrophages , lymphocytes and plasma cells
also present at the site.
- No tissue necrosis and acute inflammation.
Indications for use:
Product Presentation
|
B-OstIN Granules |
B-OstIN Rod |
B-OstIN Block |
 |
 |
 |
|
|
|